KINtaro Database
FAMILY: HopBF1 (PKLF000064)
Description
HopBF1 is a type III secretion system effector kinase. The molecular chaperone HSP90 facilitates the folding of several client proteins. HSP90 is an essential component of plant and animal immunity. Yet, pathogenic strategies that directly target the chaperone have not been described. HopBF1 family of bacterial effectors as eukaryotic-specific HSP90 protein kinases adopts a minimal protein kinase fold that is recognized by HSP90 as a host client. Consequently, HopBF1 phosphorylates HSP90 to completely inhibit the chaperone's ATPase activity. This prevents the activation of immune receptors that trigger the hypersensitive response in plants. As a result, HopBF1-dependent phosphorylation of HSP90 is sufficient to induce severe disease symptoms in plants infected with the bacterial pathogen [PMID: 31522888].
Origin
phmmer: 0.0001;
database: nr;
sequence_cutoff: 100aa;
clustering: cdhit: 90%;
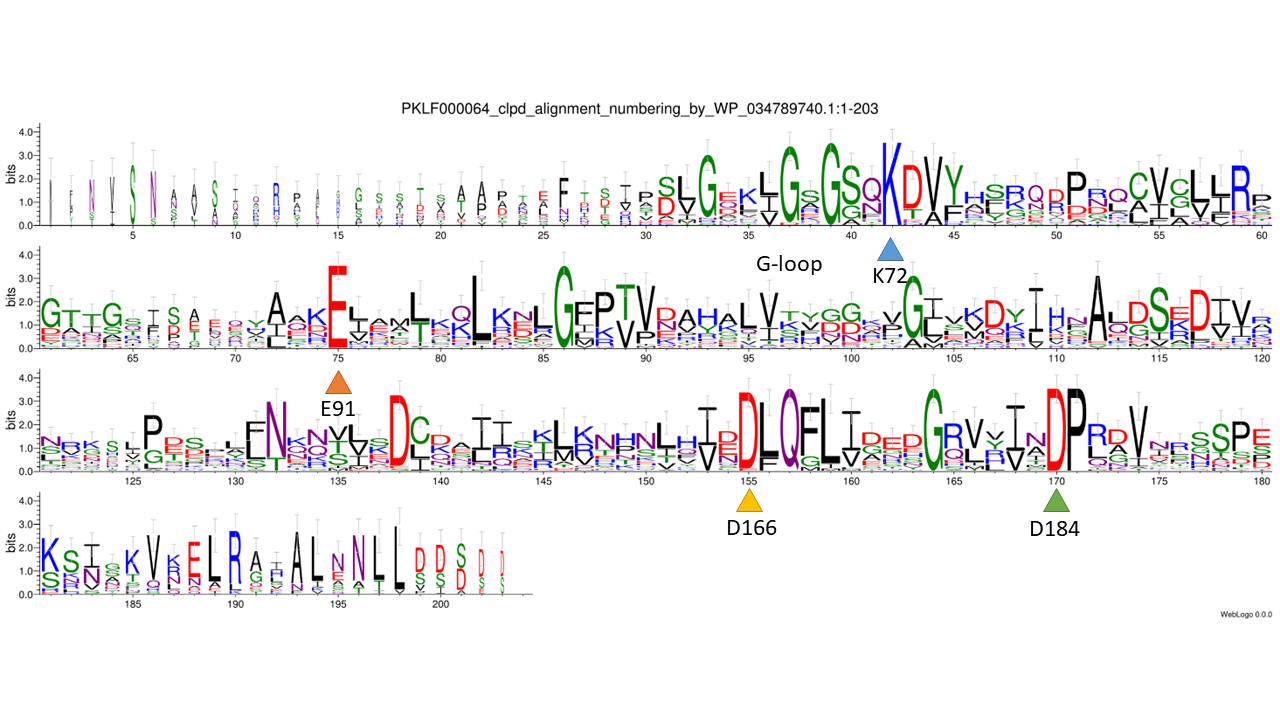
catalytic residues: unknown, only PKL fold predicted (PMID: 36917664)
Structure
PKL domain
HMM Model
WebLogo
 ×
×
![]()
Sequences
Aligned domain sequences
Download sequence
Unaligned domain sequences
Download sequence
Full sequences
Download sequence
Description
HopBF1 is a type III secretion system effector kinase. The molecular chaperone HSP90 facilitates the folding of several client proteins. HSP90 is an essential component of plant and animal immunity. Yet, pathogenic strategies that directly target the chaperone have not been described. HopBF1 family of bacterial effectors as eukaryotic-specific HSP90 protein kinases adopts a minimal protein kinase fold that is recognized by HSP90 as a host client. Consequently, HopBF1 phosphorylates HSP90 to completely inhibit the chaperone's ATPase activity. This prevents the activation of immune receptors that trigger the hypersensitive response in plants. As a result, HopBF1-dependent phosphorylation of HSP90 is sufficient to induce severe disease symptoms in plants infected with the bacterial pathogen [PMID: 31522888].
Origin
phmmer: 0.0001; database: nr; sequence_cutoff: 100aa; clustering: cdhit: 90%; catalytic residues: unknown, only PKL fold predicted (PMID: 36917664)
Structure
PKL domain
HMM Model
WebLogo
