KINtaro Database
FAMILY: Pan3_PK (PKLF000028)
Description
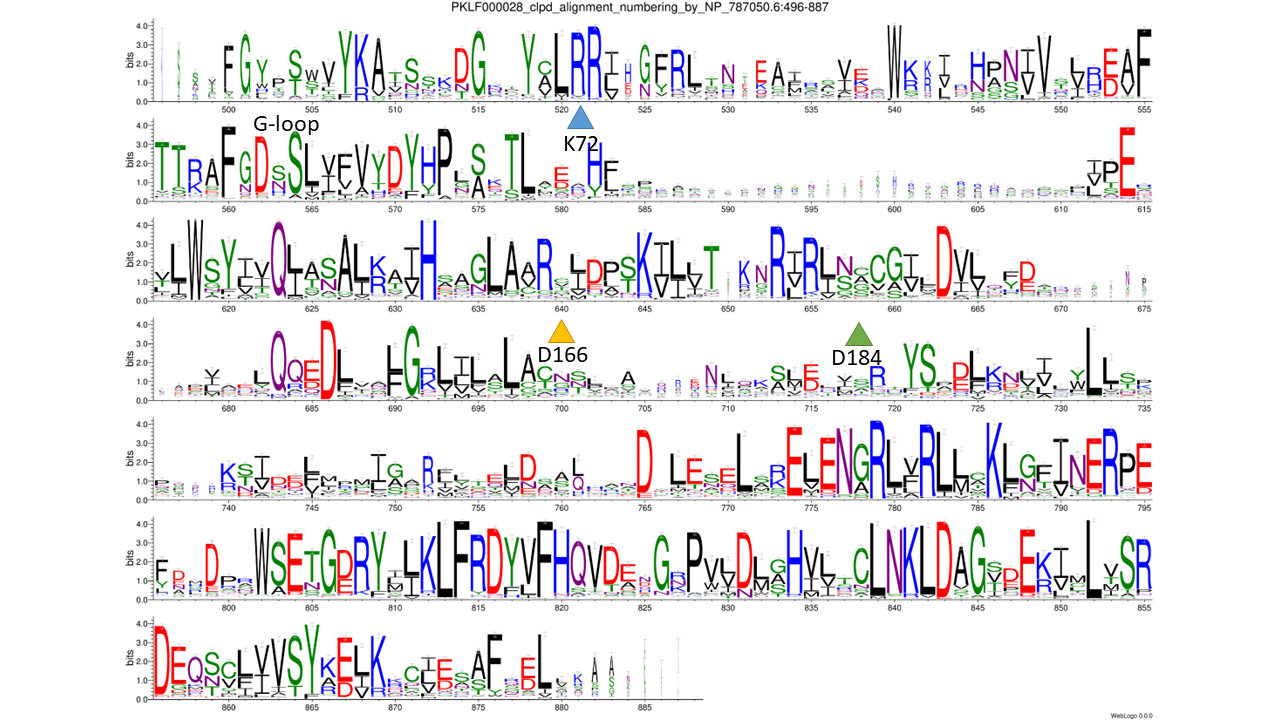
Pan3 is a pseudokinase domain found in the PAB-dependent poly(A)-specific ribonuclease subunit. Pan3 proteins consist of three main regions: an unstructured N-terminal region, a central pseudokinase domain, and a highly conserved C-terminal domain. The pseudokinase domain within Pan3 has maintained its ability to bind ATP, which is essential for mRNA degradation in vivo. Despite retaining the overall structural characteristics of protein kinases, the pseudokinase domain in Pan3 has substitutions in all the conserved motifs crucial for kinase activity. Notably, the catalytic VAIK and HRD motifs, as well as the Mg2+ binding DFG motif, have been modified. Nevertheless, the PAN3 pseudokinase domain has demonstrated an ability to bind ATP. Moreover, like other kinases, the ATP-binding site is located in the crevice between the N- and C-lobes of the kinase fold, although the ATP-binding pocket is wider than that of typical kinases [PMID: 24872509, PMID: 24880343, PMID: 24880344, PMID: 23932717].
Origin
phmmer: 0.0001;
database: nr;
sequence_cutoff: 100aa;
clustering: cdhit: 90%;
catalytic residues: based on - collapsed family logo, 3D structure model, FATCAT structural pairwise alignment with 4BWP, 1ATP, 1O6Y and 6PWD, PDB (4BWP), PMID: 23932717
Structure
PKL domain
HMM Model
WebLogo
 ×
×
![]()
Sequences
Aligned domain sequences
Download sequence
Unaligned domain sequences
Download sequence
Full sequences
Download sequence
Description
Pan3 is a pseudokinase domain found in the PAB-dependent poly(A)-specific ribonuclease subunit. Pan3 proteins consist of three main regions: an unstructured N-terminal region, a central pseudokinase domain, and a highly conserved C-terminal domain. The pseudokinase domain within Pan3 has maintained its ability to bind ATP, which is essential for mRNA degradation in vivo. Despite retaining the overall structural characteristics of protein kinases, the pseudokinase domain in Pan3 has substitutions in all the conserved motifs crucial for kinase activity. Notably, the catalytic VAIK and HRD motifs, as well as the Mg2+ binding DFG motif, have been modified. Nevertheless, the PAN3 pseudokinase domain has demonstrated an ability to bind ATP. Moreover, like other kinases, the ATP-binding site is located in the crevice between the N- and C-lobes of the kinase fold, although the ATP-binding pocket is wider than that of typical kinases [PMID: 24872509, PMID: 24880343, PMID: 24880344, PMID: 23932717].
Origin
phmmer: 0.0001; database: nr; sequence_cutoff: 100aa; clustering: cdhit: 90%; catalytic residues: based on - collapsed family logo, 3D structure model, FATCAT structural pairwise alignment with 4BWP, 1ATP, 1O6Y and 6PWD, PDB (4BWP), PMID: 23932717
Structure
PKL domain
HMM Model
WebLogo
