KINtaro Database
FAMILY: Act-Frag_cataly (PKLF000003)
Description
Actin-fragmin protein kinases are a family of enzymes that are involved in the regulation of actin dynamics, which is important for a variety of cellular processes, including cell motility, cell division, and vesicle trafficking. These enzymes are characterized by their ability to phosphorylate the actin-binding protein fragmin, which leads to changes in the structure and function of the actin cytoskeleton. LIMK1 has been shown to play a critical role in the regulation of actin dynamics in a variety of cell types. In one study, it was found that LIMK1-mediated phosphorylation of fragmin was required for the formation of stable actin filaments in fibroblasts [PMID: 11018042]. Another study demonstrated that LIMK1 was necessary for the proper formation of filopodia, which are cellular protrusions that are involved in cell migration [PMID: 9655398]. LIMK2 has been shown to have both overlapping and distinct functions compared to LIMK1. In one study, it was found that LIMK2 was required for the formation of stable actin filaments in neurons [PMID: 31319858]. Additionally, LIMK2 has been implicated in the regulation of dendritic spine morphology, which is important for synaptic plasticity and learning [PMID: 17360713].
Origin
phmmer: 0.0001;
database: nr;
sequence_cutoff: 100aa;
clustering: cdhit: 90%;
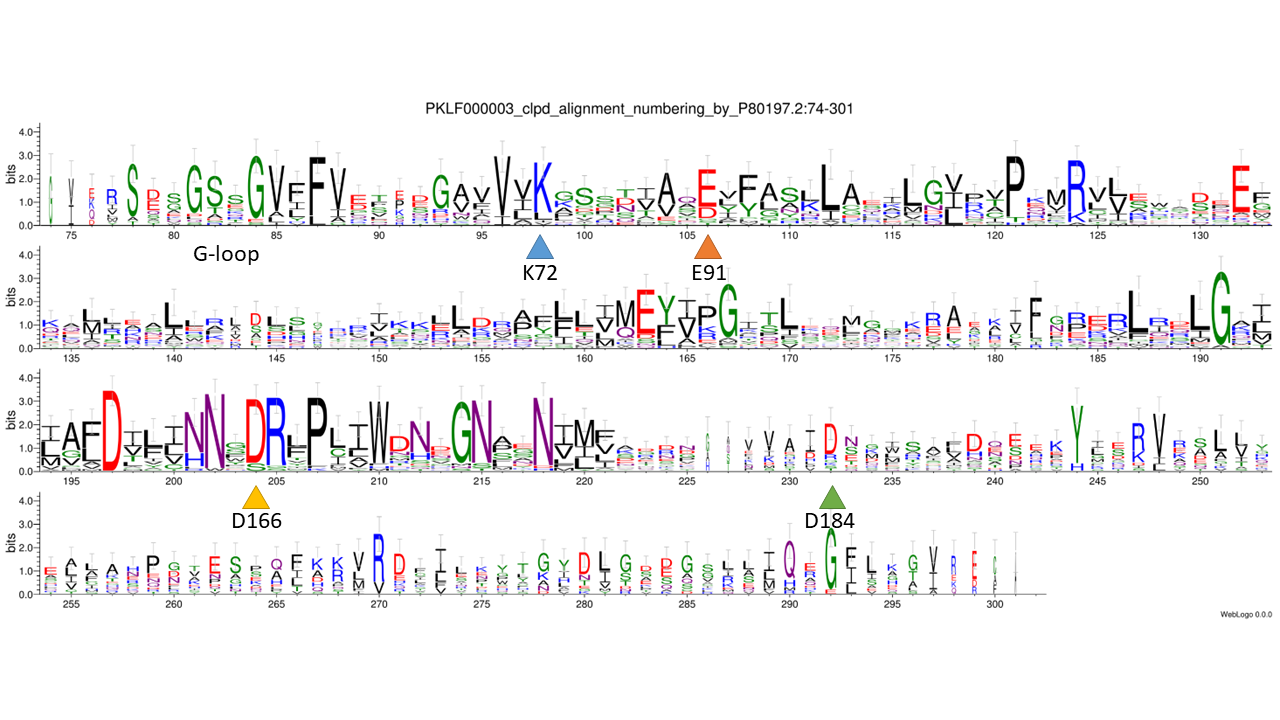
catalytic residues: based on - collapsed family logo, 3D structure model, PDB (1CJA), PMID: 10357805
Structure
PKL domain
HMM Model
WebLogo
 ×
×
![]()
Sequences
Aligned domain sequences
Download sequence
Unaligned domain sequences
Download sequence
Full sequences
Download sequence
Description
Actin-fragmin protein kinases are a family of enzymes that are involved in the regulation of actin dynamics, which is important for a variety of cellular processes, including cell motility, cell division, and vesicle trafficking. These enzymes are characterized by their ability to phosphorylate the actin-binding protein fragmin, which leads to changes in the structure and function of the actin cytoskeleton. LIMK1 has been shown to play a critical role in the regulation of actin dynamics in a variety of cell types. In one study, it was found that LIMK1-mediated phosphorylation of fragmin was required for the formation of stable actin filaments in fibroblasts [PMID: 11018042]. Another study demonstrated that LIMK1 was necessary for the proper formation of filopodia, which are cellular protrusions that are involved in cell migration [PMID: 9655398]. LIMK2 has been shown to have both overlapping and distinct functions compared to LIMK1. In one study, it was found that LIMK2 was required for the formation of stable actin filaments in neurons [PMID: 31319858]. Additionally, LIMK2 has been implicated in the regulation of dendritic spine morphology, which is important for synaptic plasticity and learning [PMID: 17360713].
Origin
phmmer: 0.0001; database: nr; sequence_cutoff: 100aa; clustering: cdhit: 90%; catalytic residues: based on - collapsed family logo, 3D structure model, PDB (1CJA), PMID: 10357805
Structure
PKL domain
HMM Model
WebLogo
