ART Database
CLADE: R-S-E (ARTC000001)
FAMILY: ADPRTs_Tse2 (ARTF000003)
Description
A family of cytoactive bacterial toxins secreted by the type VI secretory apparatus. Present mainly in Gammaproteobacteria and originally identified in Pseudomonas aeruginosa.
InterPro - IPR041018
Tse2 from P. aeruginosa has structural features similar to ADP-ribosylating toxins. It is a cytoactive toxin secreted by a type six secretion apparatus of Pseudomonas aeruginosa and found mostly in gamma proteobacteria. It naturally attacks a target in the cytoplasm of bacterial cells. Structural analysis shows similarity between Tse2 and nicotinamide adenine dinucleotide (NAD)-dependent enzymes from bacteria, notably the mono-ADP-ribosyltransferase toxins (ADPRTs). Furthermore, it revealed that the Tse2 active site is occluded upon binding the cognate immunity protein Tsi2. The abrogation of toxicity for the R14A, S80A, and H122A mutant Tse2 proteins indicates the importance of these amino acids in the mechanism of Tse2 toxicity and, given their conservation with NAD-reactive enzymes, also supports their assignment as being involved in a catalytic reaction [PUBMED:26749446].
WebLogo
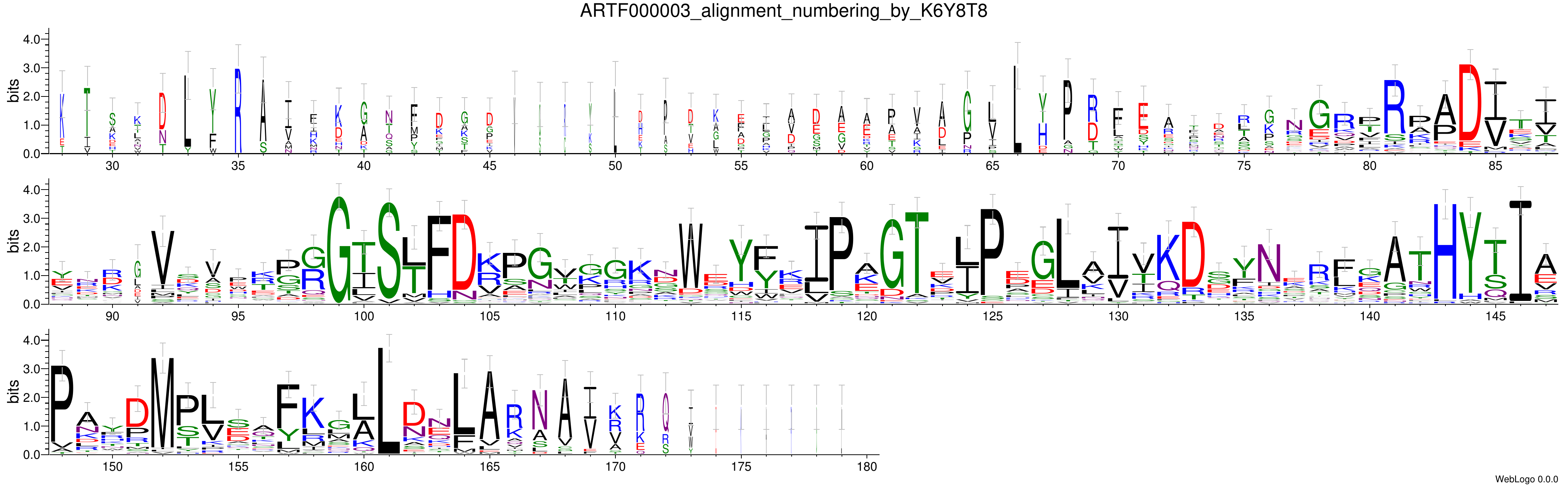
Sequences
Aligned domain sequences
Download sequenceUnaligned domain sequences
Download sequenceFull sequences
Download sequenceHMM Model
Literature
Origin
Source: Pfam 34.0, rp75 Number of sequences: 46 Average length of the domain: 107 aa HMM: Model length: 110 Clustering level: 80% Alignment: ClustalO Additional information: sequences longer than 50 amino acids
Keywords
- toxin
- cholera toxin
- Escherichia coli
- Elt
- donut-shaped pentamer
- ADP-ribosyltransferase
- heat-labile enterotoxin
- Ctx
- Vibrio cholerae
- LT